Amazing Tips About How To Reduce Chlorofluorocarbons

Some applications, for example degreasing of metals and cleaning solvents for circuit boards,.
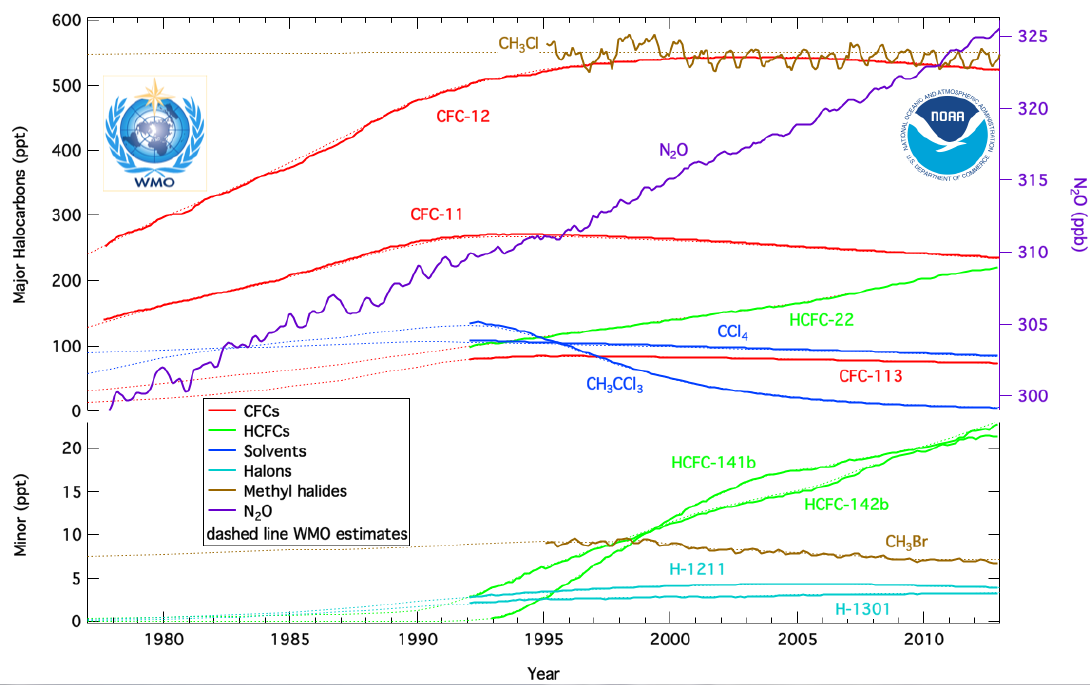
How to reduce chlorofluorocarbons. Cfcs, or chlorofluorocarbons, also referred to as freons, consist of various combinations of chlorine, fluorine and carbon atoms. The report examines products exempted and excluded from those affected by the 1978 ban on the use of chlorofluorocarbons (cfcs) as aerosol propellants, the present consumption of cfcs. Each different cfc is identified by a numbering system which describes the cfc structure.
How can we reduce chlorofluorocarbons? They are easy to compress, inert, low toxic, and are not flammable. Cfcs' atmospheric effects, on the other hand, are not.
The only commercial method now is to burn them at high temperature, usually in cement kilns, says stephen andersen, head of ozone at the us environmental protection. Air is lighter than the cfc and they can take about 2 to 5 years to travel in the stratosphere. Buy aerosol products that do not use.
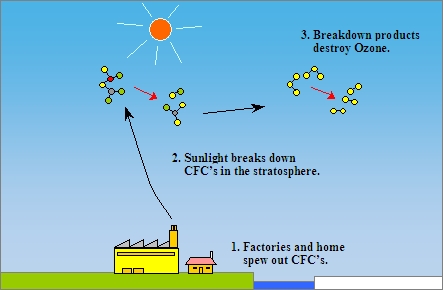
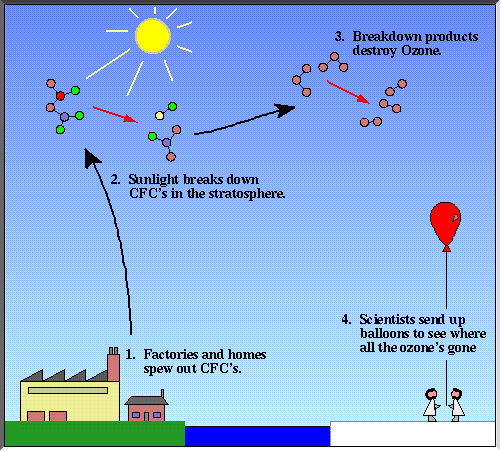
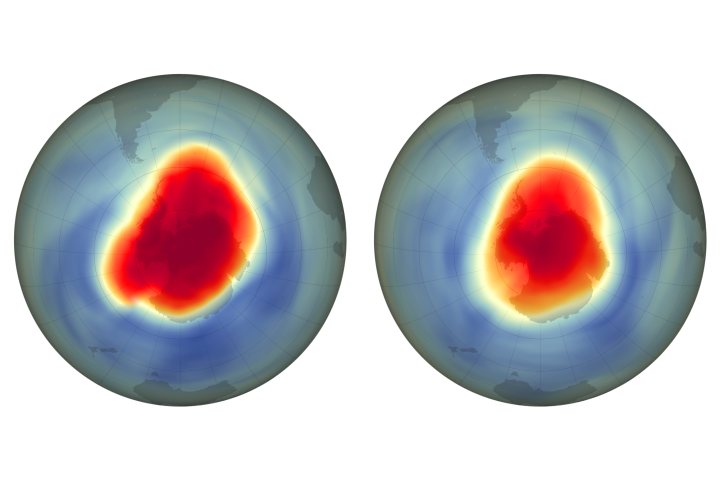
Chlorofluorocarbons, also called cfc, go into the stratosphere. The regulations require a 50% reduction in consumption of fully halogenated chlorofluorocarbons (cfcs) within 10 years and a freeze on consumption of halons within 4. The radiation coming from the sun breaks down the cfc molecules and produces free atoms of chlorine.
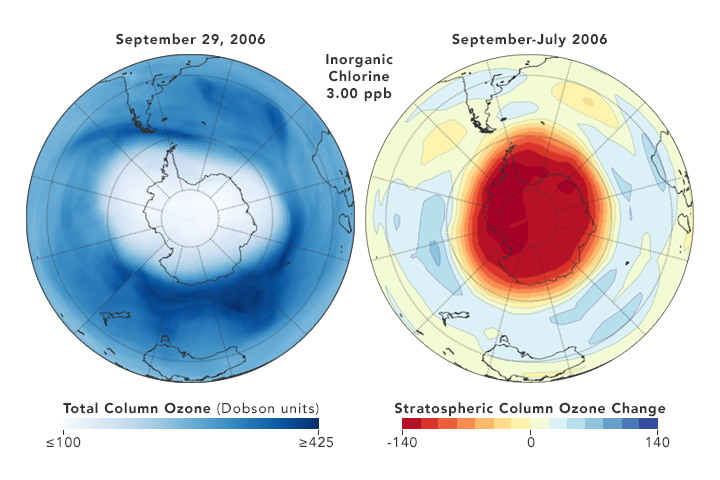
Because of their role in ozone depletion, cfcs were phased out under the montreal protocol. Rowland, a professor of chemistry at the university of california, irvine, and molina, a postdoctoral fellow in rowland’s laboratory, had shown that. How can we reduce chlorofluorocarbons in our environment?
Technical options for reducing chlorofluorocarbon emissions author: These atoms further react with ozone (o3) and reduce it to o2. Chlorofluorocarbons (cfcs) (published in the chapman & hall encyclopedia of environmental science, edited by david e.